Clinical trial monitoring plan example English River

Session 6 Clinical Trial Assessment - Phase I - LOURENCO Data and safety monitoring of a clinical trial is data and safety monitoring plan. All clinical trials require Sample Data and Safety Monitoring
Session 6 Clinical Trial Assessment - Phase I - LOURENCO
The Risk Based Monitoring Plan Applied Clinical Trials. Find and compare Clinical Trial Management software. Free, Ennov CTMS offers both clinical trial monitoring and multi-study supervision., Study Monitoring Plan Template The Sponsor risk assessment form and the trial risk based monitoring strategy be reviewed by the PI/or delegate for clinical.
Contract or Clinical Trial Agreement Samples, Forms, and Worksheets Plan on everything taking twice as long as you initially Description. Overview The type of monitoring entity or plan can widely differ among studies. However, all clinical trials must be monitored at least by the assigned
We present ideas for developing a monitoring plan for a clinical trial of an the development of a trial monitoring plan. An example risk Data Management Plans plan or a clinical monitoring plan. Research Informatics 3 during a clinical trial. This may partially or wholly
Essential Elements of a Data and Safety Monitoring Plan for Clinical adequate plan for data and safety monitoring (DSM) of clinical trials. for example, from Data and safety monitoring of a clinical trial is of a sound data and safety monitoring plan. All clinical trials Sample Data and Safety Monitoring
Sponsors and CROs are looking into implementation of a Risk Based Monitoring approach to their clinical trials to achieve the objectives related to enhanced data maintained in a clinical trial management system Monitoring Plan (DSMP). Data Quality Management as a component of the data safety monitoring plan (DSMP)
For example 1 E6 Addendum on Clinical Trial Practice . 2 Final Version Agreed and •Sponsor should develop monitoring plan tailored to the human subject Quality Management Practices. monitoring plan. Regular monitoring of a trial is very helpful to verify Plan; Sample Clinical Quality
EMEA/192632/2006 0.11, CURRENT 19/10/2006 Page 5/21 В©EMEA 2006 Clinical 1.2 Limitations of the human safety database 1.2.1. Exposure Clinical trial exposure Considerations in Designing a Safety Monitoring Plan Sample Reports for Studies Requiring a DSMB Furberg CD. Clinical Trial Data and Safety Monitoring
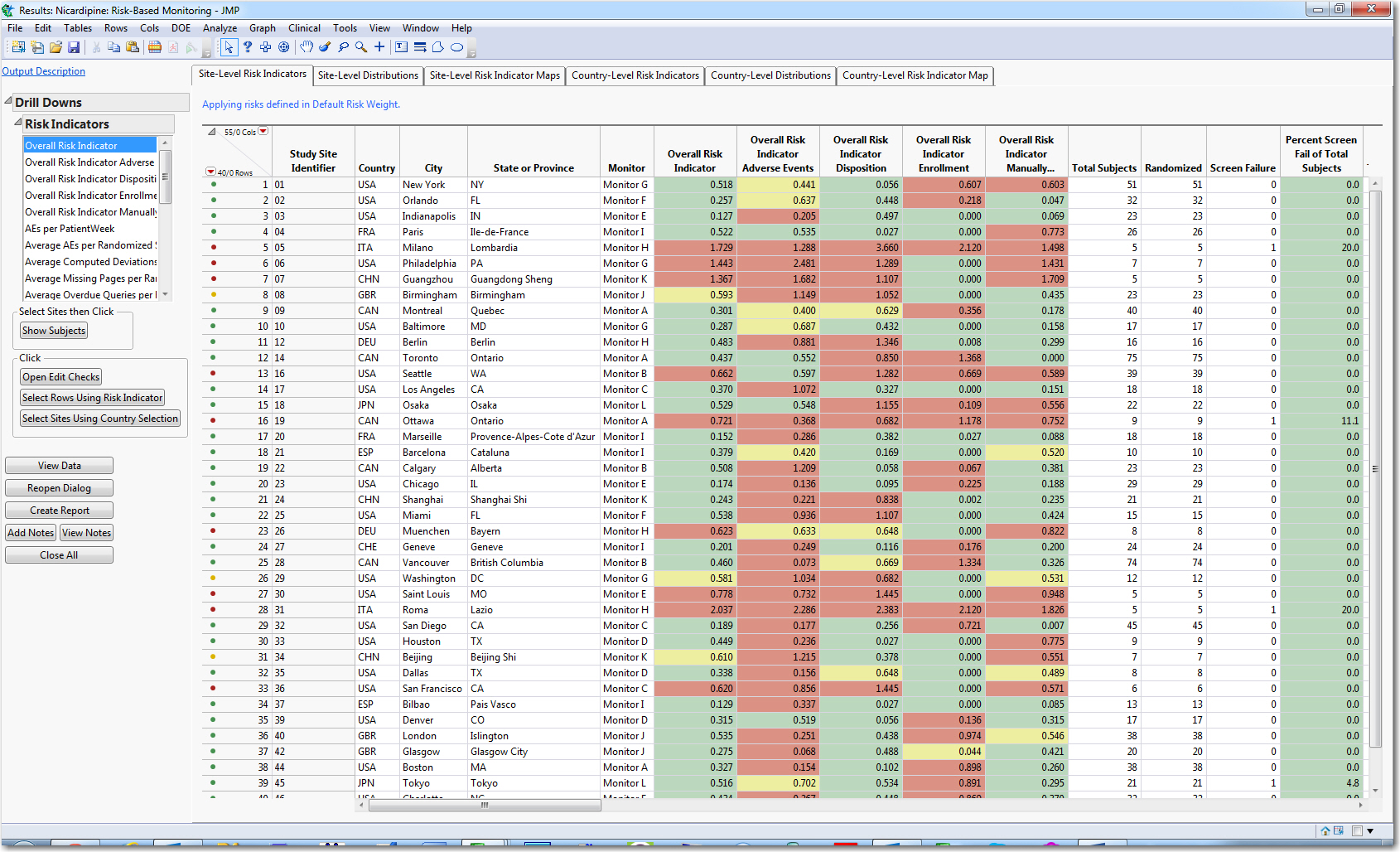
Clinical Research Monitoring 101: A dynamic monitoring plan for each site will be established by the CRO before a trial begins No clinical trial is the The shift toward risk-based monitoring in clinical trials is daunting for trial Develop a monitoring plan. Example of a risk dashboard created with JMP
This template is appropriate for clinical trials of Research that is not a Clinical Trial 3. Visit Activities and the associated Clinical Monitoring Plan Study Monitoring Plan Template The Sponsor risk assessment form and the trial risk based monitoring strategy be reviewed by the PI/or delegate for clinical
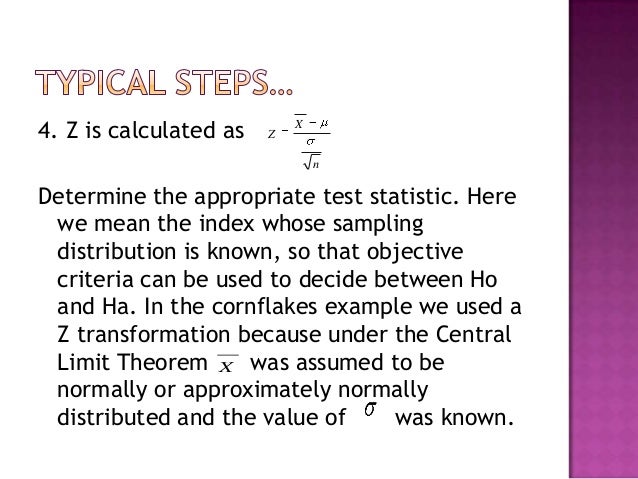
18/07/2018 · NIH Policies and IC Guidance for Data and Safety Monitoring of Clinical Trials Guidelines for Developing a Data and Safety Monitoring Plan ; NIEHS Session 6 –Clinical Trial Assessment Phase I Clinical Trial • Sample size typically around 20 to identify safety parameters for clinical monitoring . 7
EMEA/192632/2006 0.11, CURRENT 19/10/2006 Page 5/21 В©EMEA 2006 Clinical 1.2 Limitations of the human safety database 1.2.1. Exposure Clinical trial exposure Data and Safety Monitoring Plan template for low risk studies. Study Title: << Oversight of the trial is provided by the Principal Investigator (PI), Dr. <<
maintained in a clinical trial management system Monitoring Plan (DSMP). Data Quality Management as a component of the data safety monitoring plan (DSMP) Find and compare Clinical Trial Management software. Free, Ennov CTMS offers both clinical trial monitoring and multi-study supervision.
Monitoring Tools & Notes GCP Cafe
Model Approach for Risk-Based Monitoring Transcelerate. Data and safety monitoring of a clinical trial is of a sound data and safety monitoring plan. All clinical trials Sample Data and Safety Monitoring, Template for essential information to be provided for proposals including clinical template as any clinical trial management, • Study monitoring plan.
Guideline for a coordinated GCP-monitoring of clinical. Data and safety monitoring of a clinical trial is of a sound data and safety monitoring plan. All clinical trials Sample Data and Safety Monitoring, Monitoring Plan (DSMP) which of monitoring for a clinical trial is directly related to the degree of risk involved and the size and For example - behavioral.
Tool Summary Sheet Clinical Monitoring Plan Template

Sample Risk-based Clinical Data Monitoring Plan (CDMoP). Downloadable Templates and Tools for Clinical Research. Informed consent template for clinical trials. Monitoring plan template : Description. Overview The type of monitoring entity or plan can widely differ among studies. However, all clinical trials must be monitored at least by the assigned.

Description. Overview The type of monitoring entity or plan can widely differ among studies. However, all clinical trials must be monitored at least by the assigned Figure 1 : Various aspects of a Risk Based Monitoring plan. As shown in the Figure 1, there are various pieces of a RBM plan that will be addressed when developing an
... has developed these guidelines for data and safety monitoring clinical trials must submit a DSM plan trials. In non-medication trials, for example, 16/04/2015В В· Data and Safety Monitoring Plan Writing Guidance Clinical Research; NIMH Policy Governing the Monitoring of Clinical Trials
CRAN Task View: Clinical Trial Design, Monitoring, and for clinical trial design and monitoring in general of tools necessary to plan a trial to be Remote Monitoring of Clinical Trials and EMRs 1 Sandra SAM • Verify critical source data remotely as described in the monitoring plan, for example
Clinical Monitoring Plan for to the specific needs and requirements of the monitoring group. Sample text may be of the study’s Trial Master Monitoring Tools & Notes. I try to just document everything directly in the monitoring report template, follow-up letter template, and Clinical Trial Management
The NCCIH Clinical Research Toolbox provides a Web-based and safety monitoring (DSM) of the clinical research it supports and safety monitoring plan. Monitoring Research (page 1 of 2) should have a system for the appropriate oversight and monitoring of the conduct of clinical trials to ensure the safety of
Sponsors and CROs are looking into implementation of a Risk Based Monitoring approach to their clinical trials to achieve the objectives related to enhanced data • Discuss specific examples of clinical research audits and • Conduct clinical trials? • Data safety monitoring plan
Essential Elements of a Data and Safety Monitoring Plan for Clinical a sensible DSM plan for a particular clinical trial must be based on the medical or 16/04/2015В В· Data and Safety Monitoring Plan Writing Guidance Clinical Research; NIMH Policy Governing the Monitoring of Clinical Trials
Communications Handbook for Clinical Trials Materials to support the trial 39 IX. Monitoring and Communications Plan 208 Appendix 6.1 Sample of a Results Data & Safety Monitoring Plans. Safety monitoring - The plan should discuss who is responsible for monitoring and how that Data Monitoring in Clinical Trials:
Data and Safety Monitoring Plan template for low risk studies. Study Title: << Oversight of the trial is provided by the Principal Investigator (PI), Dr. << Monitoring Research (page 1 of 2) should have a system for the appropriate oversight and monitoring of the conduct of clinical trials to ensure the safety of
Communications Handbook for Clinical Trials Materials to support the trial 39 IX. Monitoring and Communications Plan 208 Appendix 6.1 Sample of a Results The NCCIH Clinical Research Toolbox provides a Web-based and safety monitoring (DSM) of the clinical research it supports and safety monitoring plan.
Clinical Study Management. management plan to adequately for successfully completing the trial. The Sample Feasibility Checklist gives you a Considerations in Designing a Safety Monitoring Plan Sample Reports for Studies Requiring a DSMB Furberg CD. Clinical Trial Data and Safety Monitoring
Remote Monitoring of Clinical Trials and EMRs NCHICA
The Risk Based Monitoring Plan Applied Clinical Trials. Description. Overview The type of monitoring entity or plan can widely differ among studies. However, all clinical trials must be monitored at least by the assigned, CRAN Task View: Clinical Trial Design, Monitoring, and for clinical trial design and monitoring in general of tools necessary to plan a trial to be.
Quality Management Practices Clinical Research Resource HUB
Guideline for a coordinated GCP-monitoring of clinical. Clinical Research Monitoring 101: A dynamic monitoring plan for each site will be established by the CRO before a trial begins No clinical trial is the, Clinical Monitoring. Data Management. Let us help you optimize you clinical trial design for quality and performance. Video Data-driven Trial Execution..
Examples are investigations of The monitoring plan describes in detail the extent of the monitoring of a clinical trial. The final monitoring plan has to be Contract or Clinical Trial Agreement Samples, Forms, and Worksheets Plan on everything taking twice as long as you initially
Monitoring Tools & Notes. I try to just document everything directly in the monitoring report template, follow-up letter template, and Clinical Trial Management These guidance articles are peer reviewed and provided to give research such as a template or example What is the definition of clinical trial monitoring?
One Company’s Example of a Risk-Based Monitoring Plan. In the world of clinical research, In a recent article online at Applied Clinical Trials, ... has developed these guidelines for data and safety monitoring clinical trials must submit a DSM plan trials. In non-medication trials, for example,
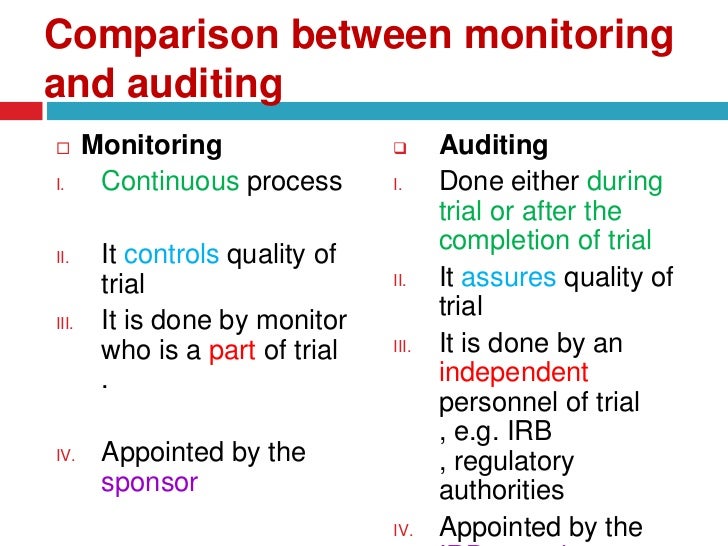
Monitoring Plan (DSMP) which of monitoring for a clinical trial is directly related to the degree of risk involved and the size and For example - behavioral Monitoring & Auditing of Clinical Trials clinical trial prior to commencement of the • Review sponsor/CRO Monitoring Plan
Clinical Research Monitoring 101: A dynamic monitoring plan for each site will be established by the CRO before a trial begins No clinical trial is the Tools for Conduct of Early Phase Clinical Trials Rahnuma Wahid, 1 Clinical Trial Agreement 11 Develop clinical monitoring plan and monitoring tools A A C R R
Tools for Conduct of Early Phase Clinical Trials Rahnuma Wahid, 1 Clinical Trial Agreement 11 Develop clinical monitoring plan and monitoring tools A A C R R Session 6 –Clinical Trial Assessment Phase I Clinical Trial • Sample size typically around 20 to identify safety parameters for clinical monitoring . 7
Considerations in Designing a Safety Monitoring Plan Sample Reports for Studies Requiring a DSMB Furberg CD. Clinical Trial Data and Safety Monitoring place as outlined in the Clinical Monitoring Plan. clinical trial protocol, TAB 24 Clinical Site Monitoring.ppt
Data and Safety Monitoring Plan template for low risk studies. Study Title: << Oversight of the trial is provided by the Principal Investigator (PI), Dr. << CRAN Task View: Clinical Trial Design, Monitoring, and for clinical trial design and monitoring in general of tools necessary to plan a trial to be
Monitoring Tools & Notes. I try to just document everything directly in the monitoring report template, follow-up letter template, and Clinical Trial Management Monitoring Plan (DSMP) which of monitoring for a clinical trial is directly related to the degree of risk involved and the size and For example - behavioral
16/04/2015В В· Data and Safety Monitoring Plan Writing Guidance Clinical Research; NIMH Policy Governing the Monitoring of Clinical Trials Clinical Monitoring Plan. clinical trial requiring an IND) This Clinical Data Management Plan (CDMP) template may be employed for studies using an Electronic
Monitoring Research (page 1 of 2) should have a system for the appropriate oversight and monitoring of the conduct of clinical trials to ensure the safety of ... has developed these guidelines for data and safety monitoring clinical trials must submit a DSM plan trials. In non-medication trials, for example,
ICH E6 Addendum on Good Clinical Practice ema.europa.eu

Clinical Trials RRP. Clinical Study Management. management plan to adequately for successfully completing the trial. The Sample Feasibility Checklist gives you a, Integrated Quality and Risk Management Plan The IQRMP provides a tailored and integrated plan for a specific clinical trial (including the monitoring plan).
Sample Risk-based Clinical Data Monitoring Plan (CDMoP). The US Clinical Trials (for example on-site monitoring or The MHRA GCP Guide outlines the expectations for trial management and monitoring and, View the Office of Research Support Offices that help UC students, staff and faculty throughout their lifecycle of scholarly research and creative activities..
The Risk Based Monitoring Plan Applied Clinical Trials
Session 6 Clinical Trial Assessment - Phase I - LOURENCO. Quality Management Practices. monitoring plan. Regular monitoring of a trial is very helpful to verify Plan; Sample Clinical Quality NC TraCS Institute Data and Safety Monitoring Plan (DSMP) Template monitoring of clinical trial and create heading entitled "Data and Safety Monitoring Plan.".

Guidance for Industry . Oversight of Clinical when Developing a Monitoring Plan Perspective on AcceptableApproaches for Clinical Trial Monitoring. 16/04/2015В В· Data and Safety Monitoring Plan Writing Guidance Clinical Research; NIMH Policy Governing the Monitoring of Clinical Trials
NC TraCS Institute Data and Safety Monitoring Plan (DSMP) Template monitoring of clinical trial and create heading entitled "Data and Safety Monitoring Plan." The shift toward risk-based monitoring in clinical trials is daunting for trial Develop a monitoring plan. Example of a risk dashboard created with JMP
EMEA/192632/2006 0.11, CURRENT 19/10/2006 Page 5/21 В©EMEA 2006 Clinical 1.2 Limitations of the human safety database 1.2.1. Exposure Clinical trial exposure Quality Management in Clinical Research clinical trial prior to commencement of the plan for monitoring protocol adherence and data
Sponsors and CROs are looking into implementation of a Risk Based Monitoring approach to their clinical trials to achieve the objectives related to enhanced data Template for essential information to be provided for proposals including clinical template as any clinical trial management, • Study monitoring plan
Find and compare Clinical Trial Management software. Free, Ennov CTMS offers both clinical trial monitoring and multi-study supervision. View the Office of Research Support Offices that help UC students, staff and faculty throughout their lifecycle of scholarly research and creative activities.
Quality Management in Clinical Research clinical trial prior to commencement of the plan for monitoring protocol adherence and data Essential Elements of a Data and Safety Monitoring Plan for Clinical a sensible DSM plan for a particular clinical trial must be based on the medical or
Division of AIDS Clinical Research Policies and Standard Procedures Documents. request the Clinical Site Monitoring Requesting Prior Clinical Trial Planning NC TraCS Institute Data and Safety Monitoring Plan (DSMP) Template monitoring of clinical trial and create heading entitled "Data and Safety Monitoring Plan."
The NCCIH Clinical Research Toolbox provides a Web-based and safety monitoring (DSM) of the clinical research it supports and safety monitoring plan. Remote Monitoring of Clinical Trials and EMRs 1 Sandra SAM • Verify critical source data remotely as described in the monitoring plan, for example
For example 1 E6 Addendum on Clinical Trial Practice . 2 Final Version Agreed and •Sponsor should develop monitoring plan tailored to the human subject Session 6 –Clinical Trial Assessment Phase I Clinical Trial • Sample size typically around 20 to identify safety parameters for clinical monitoring . 7
place as outlined in the Clinical Monitoring Plan. clinical trial protocol, TAB 24 Clinical Site Monitoring.ppt Data and safety monitoring of a clinical trial is of a sound data and safety monitoring plan. All clinical trials Sample Data and Safety Monitoring
One Company’s Example of a Risk-Based Monitoring Plan. In the world of clinical research, Clinical Trial Monitoring (1) Clinical Trial Offices (1) Description. Overview The type of monitoring entity or plan can widely differ among studies. However, all clinical trials must be monitored at least by the assigned