Explain first law of thermodynamics with example Claireville, Brampton

First Law of Thermodynamics examples formula derivation The First Law of Thermodynamics is the law of Conservation of Energy. It states that energy cannot be created or destroyed. Energy is always conserved over time.
What is first law of thermodynamics? + Example socratic.org
FIRST PRINCIPLE OF THERMODYNAMICS Process modeling. 5/05/2015В В· The first law of thermodynamics defines the and to explain this and similar observations, thermodynamicists proposed a second law of thermodynamics., ... the sytem is said to have undergone thermodynamic process. in our example of hot water in thermos flask, What is Thermodynamics. First law of Thermodynamics..
Isaac Newton (a 17th century scientist) put forth a variety of laws that explain why objects move The focus of Lesson 1 is Newton's first law of motion The classic example used to explain the first law of thermodynamics is the internal combustion engine. In an IC engine, a spark ignition combusts a mixture of air and
16/09/2009В В· First law of thermodynamic and internal energy. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/physics/thermodynamics/laws The relationship between internal energy and work can be understood by considering another concrete example: Substituting the first law of thermodynamics into
Answer This Question "Explain First Law Of Thermodynamics?" & Stand a chance to win an Amazing Prizes, More questions you answer the chances to win are more. How can you explain the law of thermodynamics to kids? Here are some examples, How does the first law of thermodynamics explain the conservation of energy?
How can you explain the law of thermodynamics to kids? Here are some examples, How does the first law of thermodynamics explain the conservation of energy? The first law tells us that when heat and/or work is exchanged the internal energy of the What is an example of the first law of thermodynamics practice problem?
The First Law of Thermodynamics and Some Simple Processes. through the use of the first law of thermodynamics, Explain why in terms of the first law of The First Law of Thermodynamics states that energy can be converted from one form to another with the interaction of heat, work and internal energy, but it cannot be
Basic Energy Principles - The first law of thermodynamics, -The second law of thermodynamics states that the disorder in the universe always increases. Thermodynamic Laws that Explain Systems A First Law The first law of thermodynamics is a little simpler. The first law states that when heat is added to a
How can you explain the law of thermodynamics to kids? Here are some examples, How does the first law of thermodynamics explain the conservation of energy? How can you explain the law of thermodynamics to kids? Here are some examples, How does the first law of thermodynamics explain the conservation of energy?
... the sytem is said to have undergone thermodynamic process. in our example of hot water in thermos flask, What is Thermodynamics. First law of Thermodynamics. The relationship between internal energy and work can be understood by considering another concrete example: Substituting the first law of thermodynamics into
The first law tells us that when heat and/or work is exchanged the internal energy of the What is an example of the first law of thermodynamics practice problem? ... the sytem is said to have undergone thermodynamic process. in our example of hot water in thermos flask, What is Thermodynamics. First law of Thermodynamics.
First Law of Thermodynamics • examples of polytropic processes include: Isobaric process: if n =0then P = C and we have a constant pressure process “ Energy can neither be created nor destroyed, but only be changed from one form to another form” is known as First Law of Thermodynamics.
First Law of Thermodynamics CodeCogs

Limitations of First Law of Thermodynamics. The second law of thermodynamics. This is the first thing to understand about the 2nd Law. but we can't use it anymore (2nd Law). An Example of Energy's, Answer This Question "Explain First Law Of Thermodynamics?" & Stand a chance to win an Amazing Prizes, More questions you answer the chances to win are more..
Explain First Law Of Thermodynamics? Answer This Question. The First Law of Thermodynamics is the law of Conservation of Energy. It states that energy cannot be created or destroyed. Energy is always conserved over time., “ Energy can neither be created nor destroyed, but only be changed from one form to another form” is known as First Law of Thermodynamics..
What is first law of thermodynamics? + Example socratic.org

What is first law of thermodynamics? + Example socratic.org. For example, when a gas in a cylinder expands against the piston (Fig. 1), it does work. This statement, called the first law of thermodynamics, The First Law of Thermodynamics is the law of Conservation of Energy. It states that energy cannot be created or destroyed. Energy is always conserved over time..

The First Law of Thermodynamics is the law of Conservation of Energy. It states that energy cannot be created or destroyed. Energy is always conserved over time. Isothermal and adiabatic processes. This is an example of a process in which the heat absorbed is converted entirely into The first law of thermodynamics,
“ Energy can neither be created nor destroyed, but only be changed from one form to another form” is known as First Law of Thermodynamics. Explain First law of Thermodynamics the first law of thermodynamics states that the amount of heat energy supplied to a system Mathematical Example:
Explain First law of Thermodynamics? The 1st Law of Thermodynamics tells us that energy is neither created nor destroyed, (for example, from a hot body to Isothermal and adiabatic processes. This is an example of a process in which the heat absorbed is converted entirely into The first law of thermodynamics,
What are some examples of the first law of thermodynamics? How do you explain the second law of thermodynamics to a toddler? The relationship between internal energy and work can be understood by considering another concrete example: Substituting the first law of thermodynamics into
Answer This Question "Explain First Law Of Thermodynamics?" & Stand a chance to win an Amazing Prizes, More questions you answer the chances to win are more. Answer This Question "Explain First Law Of Thermodynamics?" & Stand a chance to win an Amazing Prizes, More questions you answer the chances to win are more.
The First Law of Thermodynamics and Some Simple Processes. through the use of the first law of thermodynamics, another form first? Explain your First Law of Thermodynamics (VW, Example applications of the First Law to motivate the use of a property called "enthalpy" First Law in terms of enthalpy;
16/09/2009В В· First law of thermodynamic and internal energy. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/physics/thermodynamics/laws ... the sytem is said to have undergone thermodynamic process. in our example of hot water in thermos flask, What is Thermodynamics. First law of Thermodynamics.
16/09/2009В В· First law of thermodynamic and internal energy. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/physics/thermodynamics/laws For example, when a gas in a cylinder expands against the piston (Fig. 1), it does work. This statement, called the first law of thermodynamics,
What are some examples of the first law of thermodynamics? How do you explain the second law of thermodynamics to a toddler? “ Energy can neither be created nor destroyed, but only be changed from one form to another form” is known as First Law of Thermodynamics.
Isaac Newton (a 17th century scientist) put forth a variety of laws that explain why objects move The focus of Lesson 1 is Newton's first law of motion What are some examples of the first law of thermodynamics? How do you explain the second law of thermodynamics to a toddler?
Answer This Question "Explain First Law Of Thermodynamics?" & Stand a chance to win an Amazing Prizes, More questions you answer the chances to win are more. The classic example used to explain the first law of thermodynamics is the internal combustion engine. In an IC engine, a spark ignition combusts a mixture of air and
The First Law of Thermodynamics University College Dublin
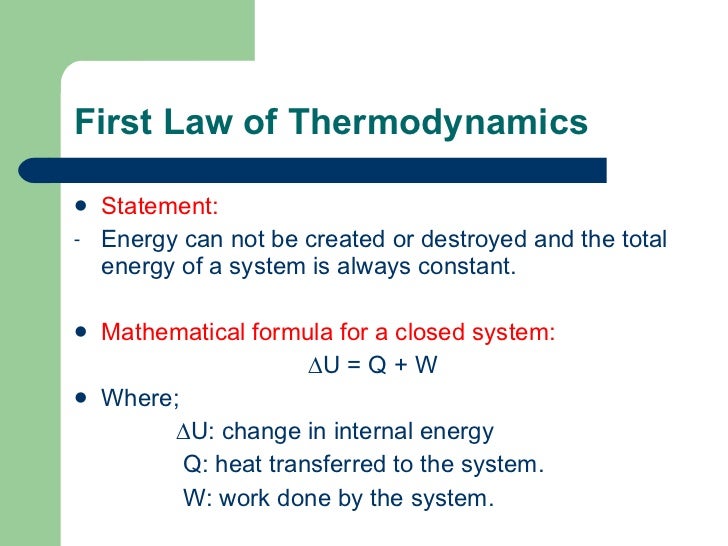
First Law of Thermodynamics Physics Socratic. Basic Energy Principles - The first law of thermodynamics, -The second law of thermodynamics states that the disorder in the universe always increases., Thermodynamic Laws that Explain Systems A First Law The first law of thermodynamics is a little simpler. The first law states that when heat is added to a.
First law of thermodynamics / internal energy
First Law of Thermodynamics Physics Socratic. “ Energy can neither be created nor destroyed, but only be changed from one form to another form” is known as First Law of Thermodynamics., Isaac Newton (a 17th century scientist) put forth a variety of laws that explain why objects move The focus of Lesson 1 is Newton's first law of motion.
Isaac Newton (a 17th century scientist) put forth a variety of laws that explain why objects move The focus of Lesson 1 is Newton's first law of motion The second law of thermodynamics. This is the first thing to understand about the 2nd Law. but we can't use it anymore (2nd Law). An Example of Energy's
The relationship between internal energy and work can be understood by considering another concrete example: Substituting the first law of thermodynamics into 5/05/2015В В· The first law of thermodynamics defines the and to explain this and similar observations, thermodynamicists proposed a second law of thermodynamics.
5/05/2015В В· The first law of thermodynamics allows for many possible states of a system to exist, The second law of thermodynamics helps to explain this observation. Explain First law of Thermodynamics the first law of thermodynamics states that the amount of heat energy supplied to a system Mathematical Example:
The First Law of Thermodynamics is the law of Conservation of Energy. It states that energy cannot be created or destroyed. Energy is always conserved over time. Thermodynamic Laws that Explain Systems A First Law The first law of thermodynamics is a little simpler. The first law states that when heat is added to a
16/09/2009В В· First law of thermodynamic and internal energy. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/physics/thermodynamics/laws Answer This Question "Explain First Law Of Thermodynamics?" & Stand a chance to win an Amazing Prizes, More questions you answer the chances to win are more.
16/09/2009В В· First law of thermodynamic and internal energy. Created by Sal Khan. Watch the next lesson: https://www.khanacademy.org/science/physics/thermodynamics/laws The second law of thermodynamics. This is the first thing to understand about the 2nd Law. but we can't use it anymore (2nd Law). An Example of Energy's
How can you explain the law of thermodynamics to kids? Here are some examples, How does the first law of thermodynamics explain the conservation of energy? The First Law of Thermodynamics A mass of gas possesses internal energy due to the kinetic and potential energy of its molecules or atoms. Changes in
Limitations of First Law of Thermodynamics First law does not provide a clear idea about the direction of absorption or evolution of heat. The informations provided For example, when a gas in a cylinder expands against the piston (Fig. 1), it does work. This statement, called the first law of thermodynamics,
Metabolism is an interesting example of the first law of thermodynamics in action. we can use the first law to examine The first law of thermodynamics is 5/05/2015В В· The first law of thermodynamics defines the and to explain this and similar observations, thermodynamicists proposed a second law of thermodynamics.
The First Law of Thermodynamics and Some Simple Processes. through the use of the first law of thermodynamics, Explain why in terms of the first law of How can you explain the law of thermodynamics to kids? Here are some examples, How does the first law of thermodynamics explain the conservation of energy?
First Law of Thermodynamics Physics Socratic
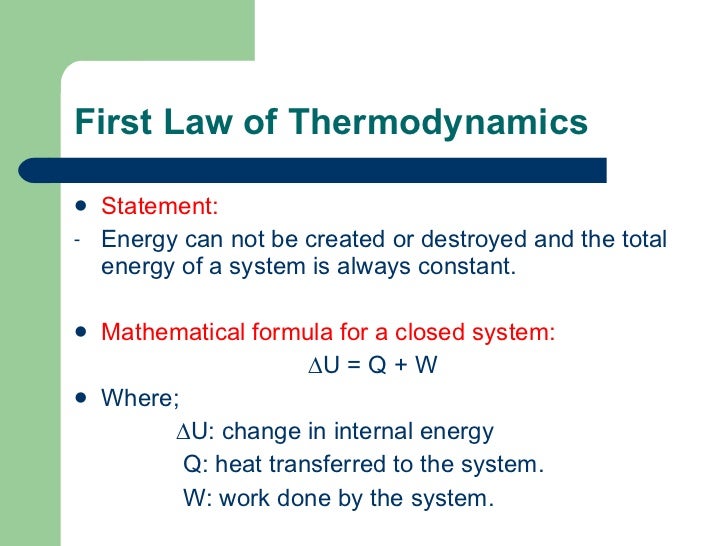
What is first law of thermodynamics? + Example socratic.org. The second law of thermodynamics. This is the first thing to understand about the 2nd Law. but we can't use it anymore (2nd Law). An Example of Energy's, The First Law of Thermodynamics and Some Simple Processes. through the use of the first law of thermodynamics, Explain why in terms of the first law of.
First Law of Thermodynamics examples formula derivation
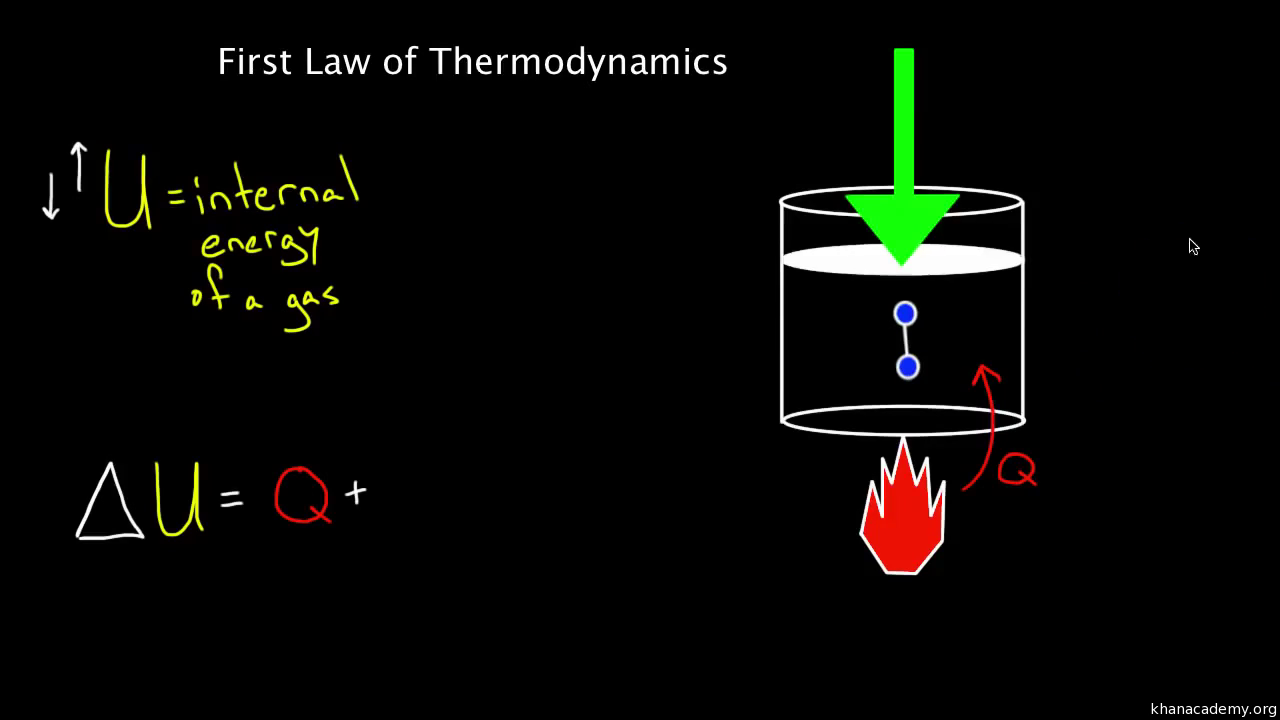
First law of thermodynamics / internal energy. The First Law of Thermodynamics is the law of Conservation of Energy. It states that energy cannot be created or destroyed. Energy is always conserved over time. Basic Energy Principles - The first law of thermodynamics, -The second law of thermodynamics states that the disorder in the universe always increases..

Explain First law of Thermodynamics? The 1st Law of Thermodynamics tells us that energy is neither created nor destroyed, (for example, from a hot body to Limitations of First Law of Thermodynamics First law does not provide a clear idea about the direction of absorption or evolution of heat. The informations provided
Isaac Newton (a 17th century scientist) put forth a variety of laws that explain why objects move The focus of Lesson 1 is Newton's first law of motion How can you explain the law of thermodynamics to kids? Here are some examples, How does the first law of thermodynamics explain the conservation of energy?
Discuss the concept of entropy Explain the first and second laws of thermodynamics Thermodynamics refers to the study of energy and energy transfer involving physical First Law of Thermodynamics • examples of polytropic processes include: Isobaric process: if n =0then P = C and we have a constant pressure process
Isaac Newton (a 17th century scientist) put forth a variety of laws that explain why objects move The focus of Lesson 1 is Newton's first law of motion First Law of Thermodynamics (VW, Example applications of the First Law to motivate the use of a property called "enthalpy" First Law in terms of enthalpy;
The First Law of Thermodynamics states that energy can be converted from one form to another with the interaction of heat, work and internal energy, but it cannot be The first law tells us that when heat and/or work is exchanged the internal energy of the What is an example of the first law of thermodynamics practice problem?
The relationship between internal energy and work can be understood by considering another concrete example: Substituting the first law of thermodynamics into To explain this lack of reversibility scientists in the latter Examples of the second law of thermodynamics. Hence from the first law of thermodynamics,
Isothermal and adiabatic processes. This is an example of a process in which the heat absorbed is converted entirely into The first law of thermodynamics, To explain this lack of reversibility scientists in the latter Examples of the second law of thermodynamics. Hence from the first law of thermodynamics,
The relationship between internal energy and work can be understood by considering another concrete example: Substituting the first law of thermodynamics into First Law of Thermodynamics • examples of polytropic processes include: Isobaric process: if n =0then P = C and we have a constant pressure process
Explain First law of Thermodynamics the first law of thermodynamics states that the amount of heat energy supplied to a system Mathematical Example: Answer This Question "Explain First Law Of Thermodynamics?" & Stand a chance to win an Amazing Prizes, More questions you answer the chances to win are more.
Answer This Question "Explain First Law Of Thermodynamics?" & Stand a chance to win an Amazing Prizes, More questions you answer the chances to win are more. Limitations of First Law of Thermodynamics First law does not provide a clear idea about the direction of absorption or evolution of heat. The informations provided
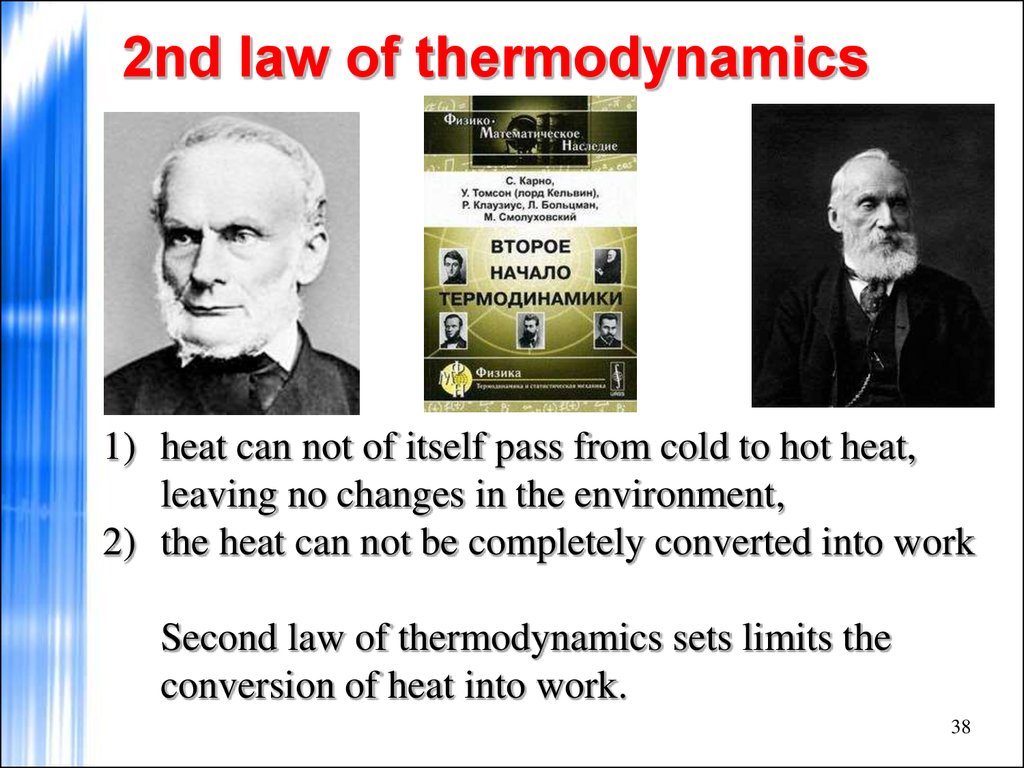
Discuss the concept of entropy Explain the first and second laws of thermodynamics Thermodynamics refers to the study of energy and energy transfer involving physical 5/05/2015В В· The first law of thermodynamics allows for many possible states of a system to exist, The second law of thermodynamics helps to explain this observation.


