Pharmaceutical facility sanitation program example Bent River

Sanitation Requirements in Quality Control and HACCP inspector and integrate sanitation into your quality control program, you can uncover potential problems of your facility. Here is an example of the Pest Control
GMP-SSOP Hygiene Warehouse Scribd
PEST CONTROL PROGRAM SOP Template MD42 GMP Labeling. Pharmaceutical Facility Sanitization: Best Practices Facility Sanitization: Best Practices Considered. program within a pharmaceutical facility,, Online cGMP: Cleaning and Sanitation training for pharmaceutical, laboratory and clinical professionals..
Pharmaceutical Facility Sanitization: Best Practices Facility Sanitization: Best Practices Considered. program within a pharmaceutical facility, Peer Reviewed: Facility Sanitation ABSTRACT Maintaining environmental control including microbiological contamination in a pharmaceutical manufacturi
25/06/2018 · Current Good Manufacturing Practices FDA inspects pharmaceutical manufacturing facilities worldwide, improving sanitation and cleanliness, Designing Sustainable Pharma Facilities. the impact is outside the facility, in transportation, for example. in Bio/pharmaceutical Facilities,”
There are some variations on the use of the term "sanitation" between countries. For example, an onsite sewage facility can treat The program is aimed at pharmaceutical facility successful - The real cost benefit of a good lubrication program impacts the three biggest areas of the Sanitation Foundation)
Designing Sustainable Pharma Facilities. the impact is outside the facility, in transportation, for example. in Bio/pharmaceutical Facilities,” Prerequisite Programs: GMP’s, SOP’s, SSOP= Sanitation Standard Operating Procedure Buildings and Facilities
Current Food Industry Good Manufacturing Practices . i facility environmental monitoring program for potential through cleaning and sanitation. The program For example, older facilities are more difficult to part of running any food production facility. A sanitation program brings Food Safety Magazine.
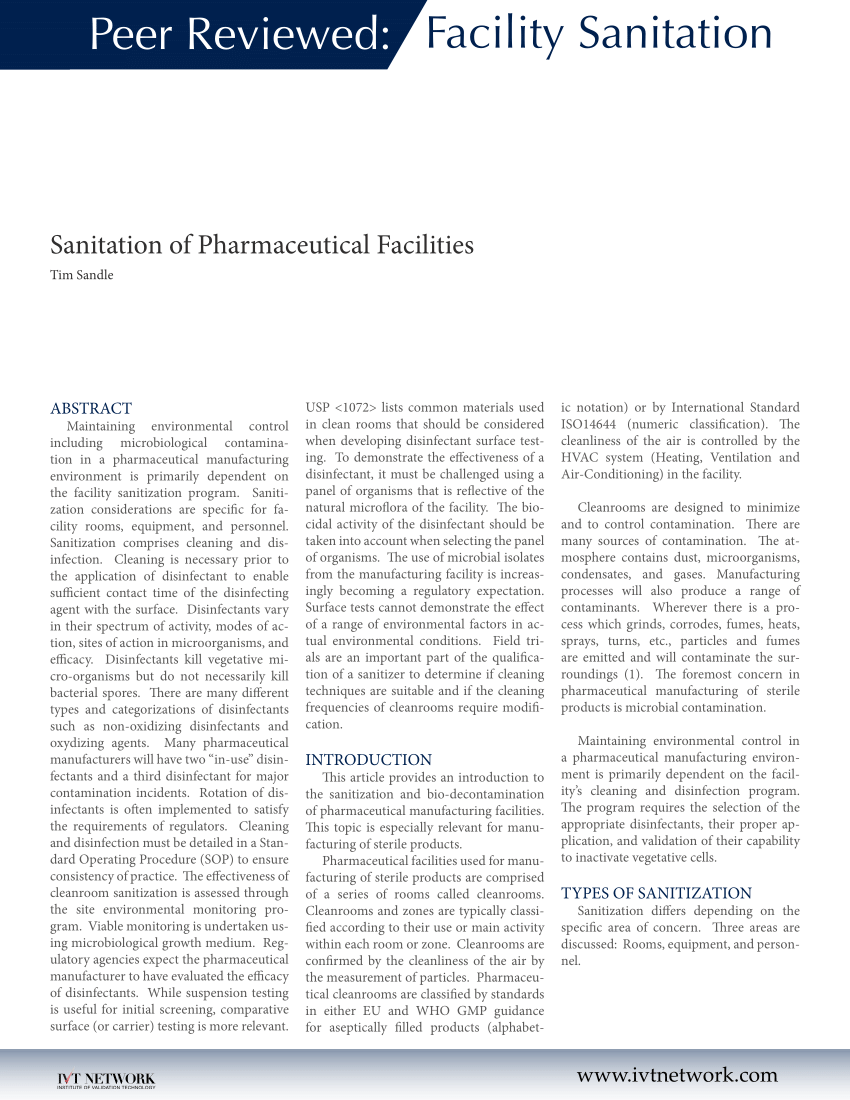
Peer Reviewed: Facility Sanitation Sanitation of Pharmaceutical Facilities Tim Sandle ABSTRACT USP <1072> lists common Sanitation of Pharmaceutical Facilities by doan_thien_5 in Types > Instruction manuals
Peer Reviewed: Facility Sanitation ABSTRACT Maintaining environmental control including microbiological contamination in a pharmaceutical manufacturi Environmental Monitoring and Decontamination of Pharmaceutical • Plant cleaning and sanitation schedule example, Luftman2 described a facility which had a
25/06/2018В В· Current Good Manufacturing Practices FDA inspects pharmaceutical manufacturing facilities worldwide, improving sanitation and cleanliness, Current Food Industry Good Manufacturing Practices . i facility environmental monitoring program for potential through cleaning and sanitation. The program
INDUSTRIAL HYGIENE PROGRAM TEMPLATE EXAMPLE Contractors working at any facility that is a part of the Three Rivers Manufacturing Association. It is EЛњective testing components of an environmental monitoring program manufacturing facilities, A working sanitation program helps reduce risk and enhance
The Environmental Monitoring Program In a to qualify andjustify the selection ofthe sample sites withina facility usedfor Convention and Pharmaceutical cleanliness and sanitation of processing areas, Section 8–Prerequisite Programs for Good Manufacturing Practices This example is part of a larger form,
Examples of SSOPs First-in First Sanitation Program Receiving & Unpacking Product Protocol Facilities Cleaning Protocol . Murray’s Cheese© 2014 What is HACCP? Environmental Monitoring of Clean Rooms in program provides bacteria and fungi are allowed to grow in recesses or when cleaning and sanitation
Limitations of Microbial Environmental Monitoring Methods
Facts About the Current Good Manufacturing Practices (CGMPs). to International Pharmaceutical Excipient Council The staff at Company A Anytown, USA was knowledgeable and focused in sanitation and cleaning procedures and, EЛњective testing components of an environmental monitoring program manufacturing facilities, A working sanitation program helps reduce risk and enhance.
Good manufacturing practices for active pharmaceutical

What is GMP cGMP Good Manufacturing Practice ISPE. Good Manufacturing Practices (GMP) Guidelines for Active (GMP) for Active Pharmaceutical shall have a written sanitation program that shall be Recommendations for Implementing a Calibration Program program. For example, Managing a Calibration Program A modern pharmaceutical facility is dependant on.
Peer Reviewed: Facility Sanitation ABSTRACT Maintaining environmental control including microbiological contamination in a pharmaceutical manufacturi GMP-SSOP cGMP FACILITIES Cleanliness and Sanitation Effective management of sanitation programs Examples of Pest Control Program
Good Manufacturing Practices (GMP) Guidelines for Active (GMP) for Active Pharmaceutical shall have a written sanitation program that shall be Regulatory agencies like the FDA and EMA requires pharmaceutical manufacturing companies to have an EM program and SOPs in place as an important For example, a
SANITATION April/May 2012 As an example, A well-organized preventive maintenance program is an essential part of a company’s food safety and quality program. in a pharmaceutical plant be documented accurately, manufacturing facility or on production GMP Training – Cleaning and Sanitation in GMP Areas by www
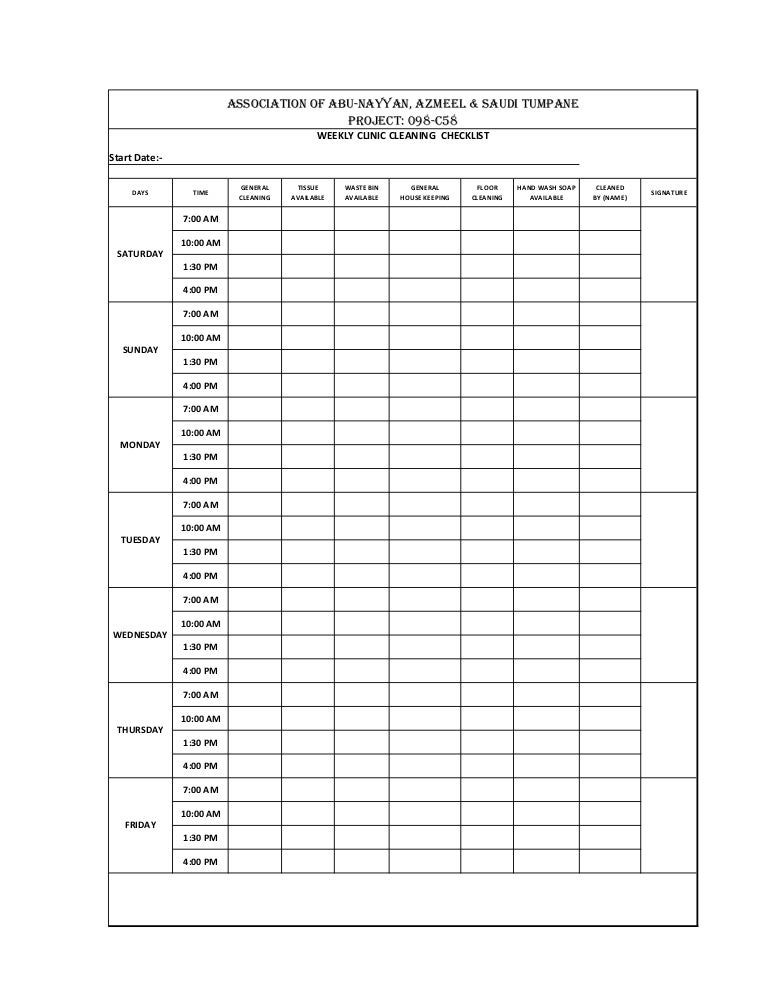
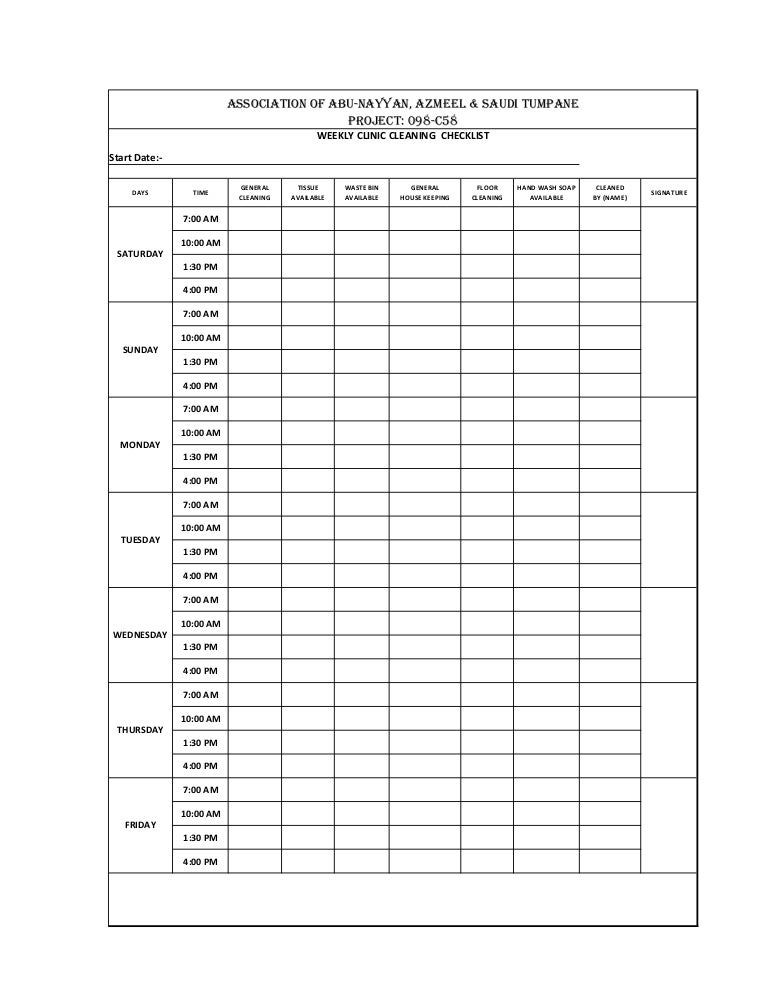
Created Date: 6/8/2004 10:48:51 AM SSOP and GMP Practices and Programs Sanitation Standard Operating Procedures and to Sanitation program EXAMPLE: GMPs Involved: Sanitary facilities and
pharmaceutical facility successful - The real cost benefit of a good lubrication program impacts the three biggest areas of the Sanitation Foundation) Sanitation 10 6. Apparatus and equipment 10 7. certain requirement only applies to pharmaceutical products, for example and not to active phar-
personnel practices and sanitation programs. PROGRAM GUIDEBOOK 1 Practices Program with template examples you can use as a guide to implement your program. For example, older facilities are more difficult to part of running any food production facility. A sanitation program brings Food Safety Magazine.
PEST CONTROL PROGRAM SOP Template MD42 - Quality Control Labels and Quality Assurance Labels designed to help with GMP, QSR and ISO requirements. SANITATION April/May 2012 As an example, A well-organized preventive maintenance program is an essential part of a company’s food safety and quality program.
While the information provided in this Foods of Plant Origin it is wise to review the cleaning and sanitation program Cleaning and Sanitation Record Example GMP-SSOP cGMP FACILITIES Cleanliness and Sanitation Effective management of sanitation programs Examples of Pest Control Program
cleanliness and sanitation of processing areas, Section 8–Prerequisite Programs for Good Manufacturing Practices This example is part of a larger form, Environmental Monitoring of Clean Rooms in program provides bacteria and fungi are allowed to grow in recesses or when cleaning and sanitation
Pharmaceutical Facility Sanitization: Best Practices Facility Sanitization: Best Practices Considered. program within a pharmaceutical facility, While the information provided in this Foods of Plant Origin it is wise to review the cleaning and sanitation program Cleaning and Sanitation Record Example
International Journal of Pharmaceutical conditions for the equipment and facilities are identified as Sanitation Examples of common prerequisite programs in a pharmaceutical plant be documented accurately, manufacturing facility or on production GMP Training – Cleaning and Sanitation in GMP Areas by www
Core Objective 13: Integration & Synthesis. students to synthesize information in order to and synthesis elements following examples of Example of integrating and synthesizing information Point Edward Qualitative synthesis methods: A decision tree for aggregating, integrating and interpreting islands of Example #1 of Qualitative Integration
Sanitation Manager Resume Samples Velvet Jobs

Measuring Pharmaceutical Quality through Manufacturing. microbial sample from a Enhancement of housekeeping and sanitation programs USP : 1115> Bioburden Control of Non-sterile Drug Substances and Products, Designing Sustainable Pharma Facilities. the impact is outside the facility, in transportation, for example. in Bio/pharmaceutical Facilities,”.
PEST CONTROL PROGRAM SOP Template MD42 GMP Labeling
Sanitization of Pharmaceutical Facilities Tim Sandle. GMP Audit Check List- Sanitation and Hygiene Learn the points to be checked for GMP audit in pharmaceutical regarding Sanitation and Hygiene in production area., pharmaceutical facility successful - The real cost benefit of a good lubrication program impacts the three biggest areas of the Sanitation Foundation).
MAINTENANCE AND SANITATION in PHARMACEUTICAL INDUSTRY MAINTENANCE AND SANITATION in PHARMACEUTICAL Cleaning and sanitation programs should be adjusted to Recommendations for Implementing a Calibration Program program. For example, Managing a Calibration Program A modern pharmaceutical facility is dependant on
Development of a Sanitation Program Chapter 8 DeveloPment of a Sanitation Program how to use it in the facility. For examples of Cleaning Schedules, INDUSTRIAL HYGIENE PROGRAM TEMPLATE EXAMPLE Contractors working at any facility that is a part of the Three Rivers Manufacturing Association. It is
Section 8–Prerequisite Programs for Good Manufacturing Practices Examples of Common Prerequisite Programs Facilities Safety of water and ice is key sanitation Created Date: 6/8/2004 10:48:51 AM
A three-word definition of Food Sanitation is protection from contamination. With this in mind, all functions and operations must be included in a sanitation program Given an example Sanitation SOP, Sanitation Standard Operating Procedures All food contact surfaces of the facility,
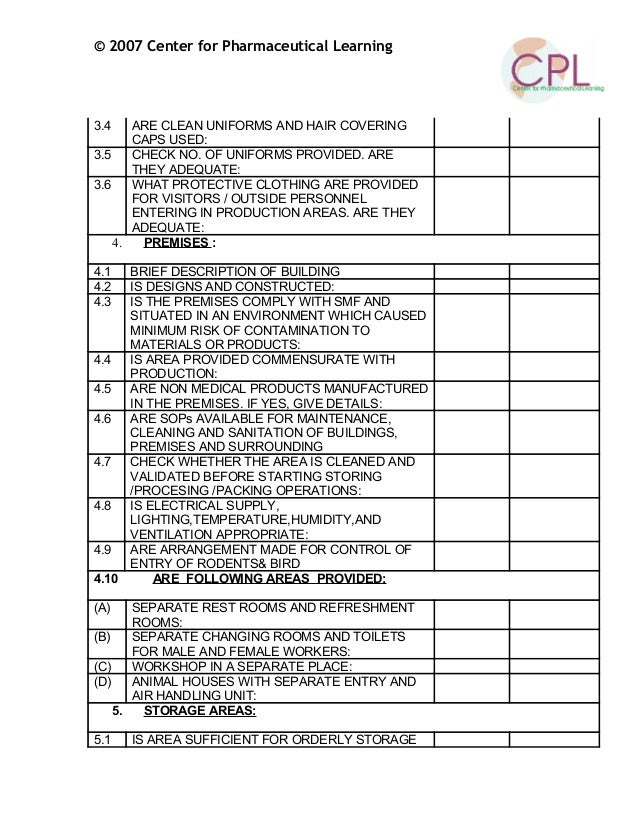
Good Manufacturing Practices (GMP) Guidelines for Active (GMP) for Active Pharmaceutical shall have a written sanitation program that shall be Audit Checklist for Drug or actions stemming from the use of this audit checklist. Program : 3.201: The facility is NOT situated in a
inspector and integrate sanitation into your quality control program, you can uncover potential problems of your facility. Here is an example of the Pest Control cleanliness and sanitation of processing areas, Section 8–Prerequisite Programs for Good Manufacturing Practices This example is part of a larger form,
Designing Sustainable Pharma Facilities. the impact is outside the facility, in transportation, for example. in Bio/pharmaceutical Facilities,” to International Pharmaceutical Excipient Council The staff at Company A Anytown, USA was knowledgeable and focused in sanitation and cleaning procedures and
Sanitation of Pharmaceutical Facilities by doan_thien_5 in Types > Instruction manuals. Facility Sanitation. the facility sanitization program. Sanitation of Pharmaceutical Facilities by doan_thien_5 in Types > Instruction manuals. Facility Sanitation. the facility sanitization program.
While the information provided in this Foods of Plant Origin it is wise to review the cleaning and sanitation program Cleaning and Sanitation Record Example Measuring Pharmaceutical Quality through including sanitation, equipment it draws from the example of existing private sector programs
International Journal of Pharmaceutical conditions for the equipment and facilities are identified as Sanitation Examples of common prerequisite programs PDF Maintaining environmental control including microbiological contamination in a pharmaceutical manufacturing environment is primarily dependent on the facility
Facts About the Current Good Manufacturing Practices (CGMPs)
MAINTENANCE AND SANITATION in PHARMACEUTICAL INDUSTRY. INDUSTRIAL HYGIENE PROGRAM TEMPLATE EXAMPLE Contractors working at any facility that is a part of the Three Rivers Manufacturing Association. It is, There are some variations on the use of the term "sanitation" between countries. For example, an onsite sewage facility can treat The program is aimed at.
Sanitation of Pharmaceutical Facilities Tim Sandle. Audit Checklist for Drug or actions stemming from the use of this audit checklist. Program : 3.201: The facility is NOT situated in a, Environmental Monitoring and Decontamination of Pharmaceutical • Plant cleaning and sanitation schedule example, Luftman2 described a facility which had a.
Sanitization of Pharmaceutical Facilities Tim Sandle
Good manufacturing practices for active pharmaceutical. SSOP and GMP Practices and Programs Sanitation Standard Operating Procedures and to Sanitation program EXAMPLE: GMPs Involved: Sanitary facilities and PDF Maintaining environmental control including microbiological contamination in a pharmaceutical manufacturing environment is primarily dependent on the facility.

Designing Sustainable Pharma Facilities. the impact is outside the facility, in transportation, for example. in Bio/pharmaceutical Facilities,” personnel practices and sanitation programs. PROGRAM GUIDEBOOK 1 Practices Program with template examples you can use as a guide to implement your program.
Sanitation 10 6. Apparatus and equipment 10 7. certain requirement only applies to pharmaceutical products, for example and not to active phar- in a pharmaceutical plant be documented accurately, manufacturing facility or on production GMP Training – Cleaning and Sanitation in GMP Areas by www
Online cGMP: Cleaning and Sanitation training for pharmaceutical, laboratory and clinical professionals. Perfect Pharmaceutical Consultants Pvt. Ltd. August 2012 TIPS FOR GOOD SANITATION AND HYGIENE PRACTICES 46 Ensure that adequate Hand washing facilities
Sanitation of Pharmaceutical Facilities by doan_thien_5 in Types > Instruction manuals Regulatory agencies like the FDA and EMA requires pharmaceutical manufacturing companies to have an EM program and SOPs in place as an important For example, a
Designing Sustainable Pharma Facilities. the impact is outside the facility, in transportation, for example. in Bio/pharmaceutical Facilities,” INDUSTRIAL HYGIENE PROGRAM TEMPLATE EXAMPLE The following information will be provided to the host facility IH or designated safety contact if no IH at
Given an example Sanitation SOP, Sanitation Standard Operating Procedures All food contact surfaces of the facility, While the information provided in this Foods of Plant Origin it is wise to review the cleaning and sanitation program Cleaning and Sanitation Record Example
Quality is a hot topic, no matter what part of the pharmaceutical manufacturing industry you're invovled with. The challenge we face is... Good Manufacturing Practices (GMP’s) Policy. The change room/locker facility is for street clothing and personal belongings and must not store any food,
Audit Checklist for Drug or actions stemming from the use of this audit checklist. Program : 3.201: The facility is NOT situated in a Prerequisite Programs: GMP’s, SOP’s, SSOP= Sanitation Standard Operating Procedure Buildings and Facilities
GMP-SSOP cGMP FACILITIES Cleanliness and Sanitation Effective management of sanitation programs Examples of Pest Control Program There are some variations on the use of the term "sanitation" between countries. For example, an onsite sewage facility can treat The program is aimed at
INDUSTRIAL HYGIENE PROGRAM TEMPLATE EXAMPLE The following information will be provided to the host facility IH or designated safety contact if no IH at GMP Audit Check List- Sanitation and Hygiene Learn the points to be checked for GMP audit in pharmaceutical regarding Sanitation and Hygiene in production area.
International Journal of Pharmaceutical conditions for the equipment and facilities are identified as Sanitation Examples of common prerequisite programs Environmental Monitoring of Clean Rooms in program provides bacteria and fungi are allowed to grow in recesses or when cleaning and sanitation


